Abstract
Background: JAK2V617F (JAK2VF) is the leading driver mutation present in hematopoietic cells of classical myeloproliferative neoplasms (MPN). The contribution of the non-hematopoietic compartment to MPN pathology remains obscure, although alterations and mutations in non-hematopoietic bone marrow (BM) cells (nonHC) can induce MPN-like diseases (reviewed in PMID32780848). Strikingly, the MPN-inducing JAK2VF mutation may be detected decades before MPN diagnosis, suggesting that the nonHC may act to support the expansion of the MPN clone. To examine this role of the nonHC within the BM during MPN disease, a model relying on low-level engraftment into an unaltered BM microenvironment is needed. Current transplantation models require myeloablative conditioning (irradiation) to ensure cell engraftment, which permanently alters the BM microenvironment (PMID24607941). We have developed a novel murine system that mimics JAK2VF+ MPN without the use of myeloablative conditioning, allowing the role of the BM microenvironment in the natural progression of MPN pathology to be studied.
Methods: Floxed-JAK2VF (JAX#031658) were crossed with Mx1-Cre (JAX#003556) mice to produce a poly I:C-inducible MPN-like disease model (CD45.2). Five-week-old donor animals, designated JAK2MUT (Mx1-Cre(+);JAK2VF(+/-)) or JAK2CTRL (Mx1-Cre(-);JAK2VF(+/-)), were injected with poly I:C to activate the JAK2VF mutation. Five weeks later, BM was isolated and transplanted via single tail vein injection into age- and sex-matched C57BL/6 Ptprca (JAX#002014, CD45.1) recipient animals, at a concentration of 1.5x107 per animal. 10 mice (5 female, 5 male) were used as BM transplant (BMT) recipients of either JAK2MUT or JAK2CTRL BM. In addition, 10 total non-injected sex- and age-matched hosts were kept as a naïve (non-injected) group. Body weight (BW) of all 20 BMT recipients was monitored as an indicator of overall health. Blood from the submandibular vein was collected from all recipients every 4-6 weeks for complete blood cell counts (CBC) and flow cytometry analysis to determine alterations within the hematopoietic system and percentage of donor (CD45.2) chimerism within CD45.1 hosts.
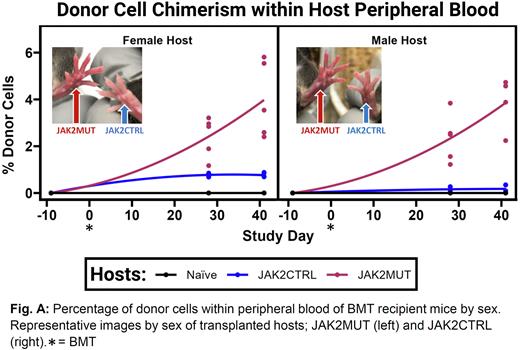
Results: At transplantation, JAK2MUT donors displayed advanced MPN-like disease (hematocrit (HCT) >70%, red blood cells (RBC) >1.9x107/µl, platelets (PLT) >1.1x106/µl). Over the course of the study, only male recipients receiving JAK2MUT donor cells showed a significantly different trend in weight change at 6 weeks post-transplantation with an average of 0.18% less BW gained. Hosts receiving JAK2CTRL cells displayed low engraftment efficiency <1% after 6 weeks post BMT, whereas hosts receiving JAK2MUT cells displayed ~4% observable engraftment within peripheral blood (Fig. A). Additionally, hosts receiving JAKMUT cells displayed reddening of extremities indictive of erythromelalgia, characteristic of JAK2VF+ MPN (Fig. A). When compared to donors, JAKMUT transplanted hosts displayed CBC abnormalities, characteristic of polycythemia (HCT >49%, RBC >25% naïve, unaltered levels of PLT) (PMID27956542). 2 of 10 recipients of JAK2MUT BMT died as the result of highly penetrant MPN 4-6 weeks after the transplant.
Conclusion: While many ways to transplant hematopoietic cells within unconditioned animals have been described, we have successfully transplanted JAK2VF BM into the non-conditioned recipients and showed presence of diagnosable disease in animals displaying ~5% donor chimerism. Our model will offer advantages over existing methods for studying early MPN disease progression within an unconditioned murine BM.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.